
In addition, constant background spontaneous hydrolysis of C3 results in formation of C3(H 2O) which also serves as a platform for the AP. Lastly, the AP is activated by C3b coming from the other two pathways. Upon recognition, the MBL-associated serine proteases (MASPs), can cleave C2 and C4, to form the C4bC2a C3 convertase ( 5– 7). These molecules recognize microbial carbohydrate structures. The LP is activated in a similar way, with ficolin and mannose-binding lectin (MBL) acting as pattern recognition molecules. C1s can then cleave C2 and C4, which leads to the formation of C3 convertase C4bC2a ( 3, 5, 6). C1q is the pattern recognition molecule, and upon surface binding of C1q, the protease C1r is activated, cleaving and activating C1s. The C1 complex consists of C1q, C1r and C1s. The CP is mainly initiated by antibody binding to target cells. With C5 convertases the terminal pathway is initiated which will result in chemotaxis and formation of the membrane attack complex (MAC) ( 3).Īlthough all three complement pathways result in the formation of a C3 convertase, their initiation and intermediate steps differ ( Figure 1). C3 convertases cleave C3, resulting in opsonization of pathogens and formation of C5 convertases.
Activation of these pathways takes place via antibody-binding, recognition of specific sugar patterns or spontaneous C3 hydrolysis, all resulting in formation of a C3 convertase. Three different pathways can initiate complement activity: the classical pathway (CP), the lectin pathway (LP) and the alternative pathway (AP). In order to respond quickly to pathogens, the components of the complement system are present in plasma and thus are readily available throughout the body ( 3, 4). Nowadays it is known that the complement system is a complex part of the innate immune system, consisting of a large number of proteins and associated regulators ( 1, 2). Upon its discovery at the end of the 19 th century, the complement system was considered to consist of only one component.

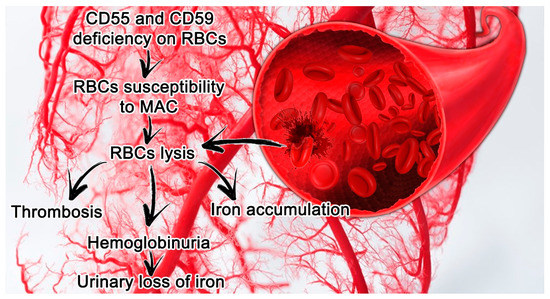
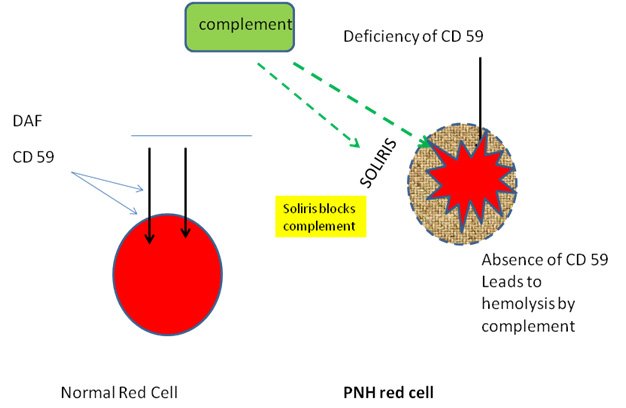
In conclusion, this review provides an overview of the complement regulatory proteins and their links to disease, together with their potential in the development of novel therapeutics. Such therapeutics are currently being developed extensively, and can be categorized into full-length complement regulators, engineered complement system regulators and antibodies targeting complement regulators. Here, we will discuss the latest insights on how complement regulators can benefit therapeutics. With further demand for therapeutics rising linked to the wide range of complement-mediated disease we should broaden our horizon towards treatments that can actually protect the host cells against complement. Currently only few drugs targeting the complement system are used in the clinic. In most of these pathologies, treatments are limited and do not prevent the complement system from attacking host cells, but rather fight the consequences of the complement-mediated damage, using for example blood transfusions in anemic patients. In addition, complement regulators have also been associated with different types of cancer, although their mechanisms here have not been elucidated yet. These diseases often result in red blood cell destruction or occur in the eye, kidney or brain, which are tissues known for aberrant complement activity or regulation. Here, we will discuss the current knowledge on complement regulatory protein polymorphisms and expression levels together with their link to disease. Their importance is indicated by the amount of pathologies associated with abnormalities in these complement regulators. To be protected from complement, each cell type relies on a specific combination of both soluble and membrane-bound regulators. Due to its strength, it is important that healthy human cells are protected against damage induced by the complement system. The complement system is an important part of the innate immune system, providing a strong defense against pathogens and removing apoptotic cells and immune complexes.


 0 kommentar(er)
0 kommentar(er)
